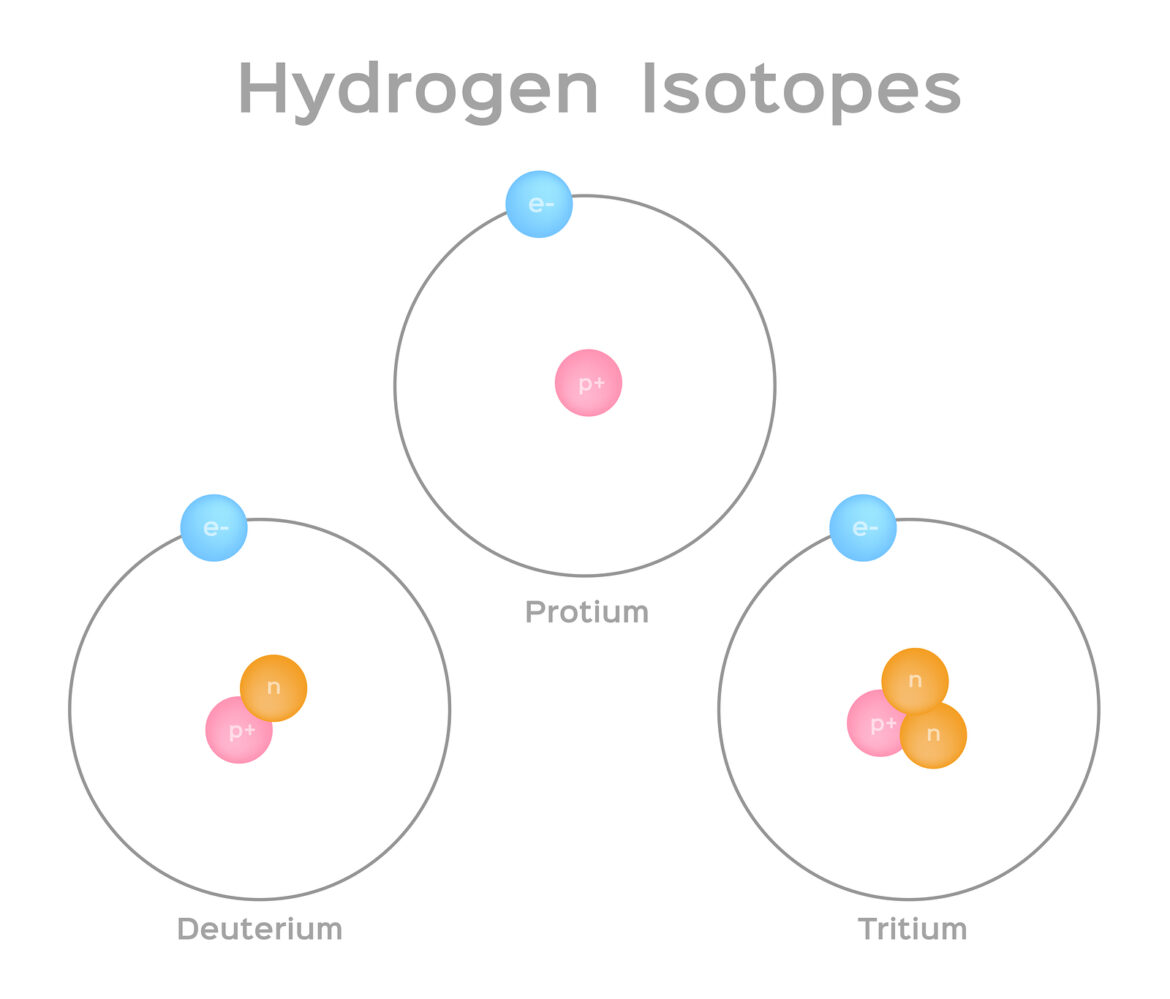
An isotope is one of two or more atoms with the same number of protons in their nuclei, but different numbers of neutrons. Isotopes are often represented by the element’s symbol followed by a superscript indicating the mass number. The number of protons determines which element an atom is, while the number of neutrons determines how stable an element is. The most common isotopes are hydrogen-1 (protium) and hydrogen-2 (deuterium), both of which have one proton in their nucleus; however, deuterium also has one neutron, making it twice as massive as protium.
The term “isotope” was first used by Frederick Soddy in 1913, when he noticed that some elements occurred in multiple forms, each with different atomic masses but identical chemical properties. He eventually determined that these atoms were not truly different elements at all, but rather versions (isotopes) of a single element with different numbers of neutrons. Today we know that every element has at least one stable isotope; some elements have hundreds!
Isotopes can be either natural or artificial (man-made). All naturally occurring uranium is radioactive because it contains three isotopes: uranium-234, uranium-235, and uranium-238. Of these three isotopes, only uranium-235 is fissile, meaning it can sustain a nuclear chain reaction. This makes it ideal for use in nuclear reactors and weapons. In contrast, plutonium-239 is an artificial radioactive isotope that is also fissile; it was created through neutron bombardment of natural uranium in a nuclear reactor. Plutonium-239 does not occur naturally because all its atom’s neutrons are unstable and decay quickly into other particles.