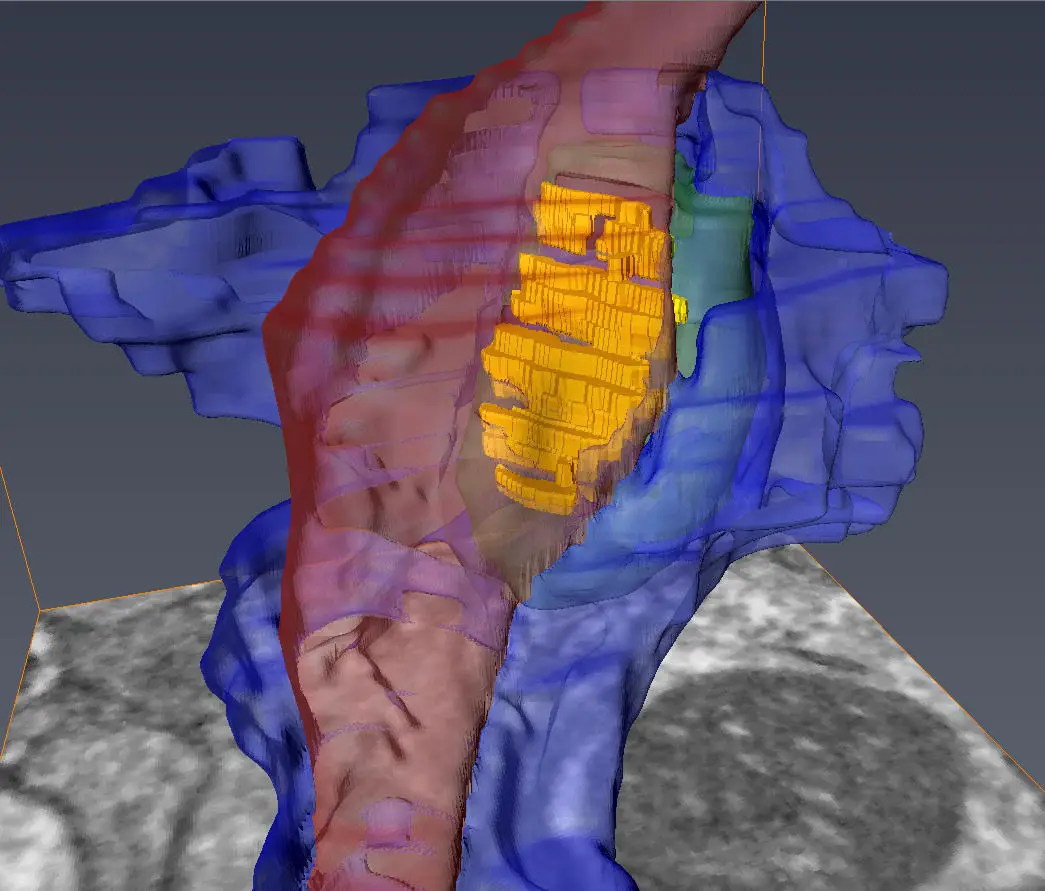
A transparent 3D model, exhibiting the axon (colored in red), medium spinal motor neuron (in green), and astrocyte (in yellow) converging at the synapse, has been made public. The credit goes to the Center for Translational Neuromedicine at the University of Rochester and University of Copenhagen.
A team of scientists has accomplished the creation of one of the most intricate 3D depictions of the synapse, which acts as a crucial junction where neurons interact with each other through chemical signal exchanges. These models, scaled at the nanometer level, will aid researchers in enhancing their comprehension and examination of neurodegenerative disorders such as Huntington’s disease and schizophrenia.
Published in the journal PNAS, the new study was headed by Steve Goldman, MD, Ph.D., the co-director of the Center for Translational Neuromedicine at the University of Rochester and the University of Copenhagen. The discoveries signify an outstanding technological advancement that enables scientists to scrutinize the diverse cells converging at individual synapses with an unprecedented level of detail.
Abdellatif Benraiss, Ph.D., a research associate professor at the Center for Translational Neuromedicine and co-author of the research, remarked, “Beyond merely understanding the synapse’s structure from written sources, it is something entirely different to witness the exact geometry of interactions between distinct cells firsthand.” He further added that this novel capability to gauge these incredibly small environments is an emerging field with the potential to further our grasp of several neurodegenerative and neuropsychiatric conditions where synaptic function is compromised.
The research team utilized this innovative technique to contrast the brains of healthy mice with those containing the mutant gene responsible for Huntington’s disease. Goldman’s prior studies had identified that malfunctioning astrocytes are instrumental in the disease’s progression. Astrocytes are part of a group of supporting cells in the brain known as glia, responsible for maintaining the appropriate chemical surroundings at the synapse.
Focusing on synapses involving medium spiny motor neurons, whose gradual depletion is a distinguishing feature of Huntington’s disease, the researchers were tasked with identifying these synapses within the complex convergence of three distinct cells: the pre-synaptic axon from a remote neuron; its target, the post-synaptic medium spiny motor neuron; and the neighboring astrocyte’s fiber processes.
To achieve this, the investigators applied viruses to allocate unique fluorescent labels to the axons, motor neurons, and astrocytes. They subsequently removed the brains, imaged the desired regions using multiphoton microscopy, and adopted a method named infrared branding with lasers to generate reference markers in the brain tissue. This enabled them to later pinpoint the cells of interest.
Utilizing a serial block-face scanning electron microscope housed at the University of Copenhagen, specifically designed to explore the brain’s minute structures, the team examined the brain tissue. This apparatus employs a diamond knife to consecutively excise and photograph ultra-thin brain tissue segments, formulating 3D models of the marked cells and their interactions at the synapse on a nanometer scale.
Carlos Benitez Villanueva, Ph.D., senior associate at the Center for Translational Neuromedicine and the study’s first author, said, “These models divulge the geometry and structural relationships between astrocytes and corresponding synapses, a critical aspect as these cells must specifically interact at the synapse.” He continued to explain that this method offers the ability to quantify and describe the geometry of the synaptic environment, even in the context of glial disease.
Examining the brains of healthy mice, the researchers noticed that the processes of astrocytes fully enclosed the space around the disk-shaped synapse, forming a secure connection. In contrast, the astrocytes in Huntington’s afflicted mice failed to adequately encase the synapse, leading to significant voids. This structural defect permits the leakage of potassium and glutamate—substances that oversee intercellular communication—potentially hindering normal cellular interactions.
The dysfunction of astrocytes has been associated with other conditions as well, including schizophrenia, amyotrophic lateral sclerosis, and frontotemporal dementia. The researchers assert that this technology could substantially enhance our knowledge of the specific structural foundation for these diseases. They specifically emphasize that this method could assess the efficacy of cell replacement strategies, substituting unhealthy glial cells with healthy ones, as a treatment for these ailments.
The article titled “Astrocytic engagement of the corticostriatal synaptic cleft is disrupted in a mouse model of Huntington’s disease” by Carlos Benitez Villanueva, Hans J. T. Stephensen, Rajmund Mokso, Abdellatif Benraiss, Jon Sporring, and Steven A. Goldman was published on 6 June 2023 in the Proceedings of the National Academy of Sciences. DOI: 10.1073/pnas.2210719120
The co-authors of the study also include Hans Stephensen and Jon Sporring from the University of Copenhagen, and Rajmund Mokso from Lund University in Sweden. The research was financially supported by the Novo Nordisk Foundation and the Lundbeck Foundation.
The user’s consent to Google Analytics and related cookies across the TrendMD network (widget, website, blog) was noted. Further information is available [here].
Table of Contents
Frequently Asked Questions (FAQs) about synapse
What are the key findings of the new neuroscience study?
The key findings of the study include the creation of one of the most detailed 3D images of the synapse, aiding researchers in understanding and studying neurodegenerative diseases like Huntington’s disease and schizophrenia. The images were achieved through a novel approach involving the use of viruses to tag different brain cells, followed by imaging using multiphoton microscopy and a serial block-face scanning electron microscope.
How does the study contribute to the understanding of neurodegenerative diseases?
The study’s intricate 3D models of the synapse reveal the geometry and structural relationships between astrocytes and synapses. This contributes to a better understanding of diseases like Huntington’s and schizophrenia, as the researchers were able to observe how structural flaws in astrocytes could disrupt normal cell-cell communication.
What techniques were used in capturing the unseen details of the synapse?
The researchers employed viruses to assign separate fluorescent tags to axons, motor neurons, and astrocytes. They then used multiphoton microscopy, infrared branding with lasers, and a serial block-face scanning electron microscope to create 3D, nanometer-scale models of the labeled cells and their interactions at the synapse.
Who were the main contributors to this research?
The study was led by Steve Goldman, MD, Ph.D., co-director of the Center for Translational Neuromedicine at the University of Rochester and the University of Copenhagen. Additional co-authors include Carlos Benitez Villanueva, Hans J. T. Stephensen, Rajmund Mokso, Abdellatif Benraiss, Jon Sporring, and researchers from Lund University in Sweden.
How might this study impact future research and treatment of neurodegenerative and neuropsychiatric diseases?
The study’s findings and techniques have the potential to advance the understanding of neurodegenerative and neuropsychiatric diseases where synaptic function is disturbed. By revealing how astrocyte dysfunction is linked to diseases like Huntington’s, ALS, and dementia, the research offers new avenues for evaluating and developing treatments, such as cell replacement strategies.
More about synapse
- Center for Translational Neuromedicine, University of Rochester
- University of Copenhagen
- Proceedings of the National Academy of Sciences (DOI link)
- Novo Nordisk Foundation
- Lundbeck Foundation