Deciphering Deafness: USC Researchers Focus on Hearing Regeneration
A person who experiences hearing loss in adulthood typically faces a permanent condition due to the inability of the inner ear’s sensory cells to regenerate after sustaining damage. Recent research, partially funded by the National Institutes of Health and documented in the Proceedings of the National Academy of Sciences (PNAS), conducted by USC Stem Cell researchers, has delved into the underlying factors behind this phenomenon and explored potential remedies.
The inner ear’s non-sensory supporting cells play a crucial role in the conversion of these cells into sensory cells. This process is disrupted through a mechanism known as “epigenetic silencing.” By studying this silencing process, researchers aim to uncover how these genes could potentially be reactivated to facilitate hearing regeneration. “Through the investigation of the shutdown process of key genes in the non-sensory cells of the inner ear, we are gaining insights into the prospect of reactivating these genes for the purpose of hearing restoration,” explained John Duc Nguyen, primary author of one of the studies. Nguyen, who was part of the USC Stem Cell laboratory led by Neil Segil, earned his Ph.D. in this domain and is currently affiliated with the biotech company Genentech.
In another study, researchers explored the temporal and procedural aspects of the inner ear’s ability to form sensory hearing cells. This study highlights two specific genes, Sox4 and Sox11, which could prove beneficial in regenerating hearing in adults. Emily Xizi Wang, the lead author of this study, underlined the rationale behind selecting these genes: “We focused on the Sox4 and Sox11 genes as they have been identified as essential for the development of sensory hearing cells during the developmental stage.” Wang conducted her research as a Ph.D. candidate in the Segil Lab and is now associated with the biotech company Atara Biotherapeutics.
Gage Crump, a co-author of both research papers and the interim chair of USC’s Department of Stem Cell Biology and Regenerative Medicine at the Keck School of Medicine of USC, emphasized the significance of these studies, not only as scientific achievements but also as a tribute to Neil Segil’s lasting influence as a mentor to the next generation of stem cell researchers.
Unveiling the Mechanisms Behind Gene Silencing
One prominent method by which genes are deactivated, known as “silencing,” involves the attachment of chemical compounds called methyl groups to DNA, rendering it inaccessible. This is the focus of Nguyen’s study. When the DNA responsible for instructing a cell to become a sensory hearing cell undergoes methylation, the cell loses the ability to access these instructive cues.
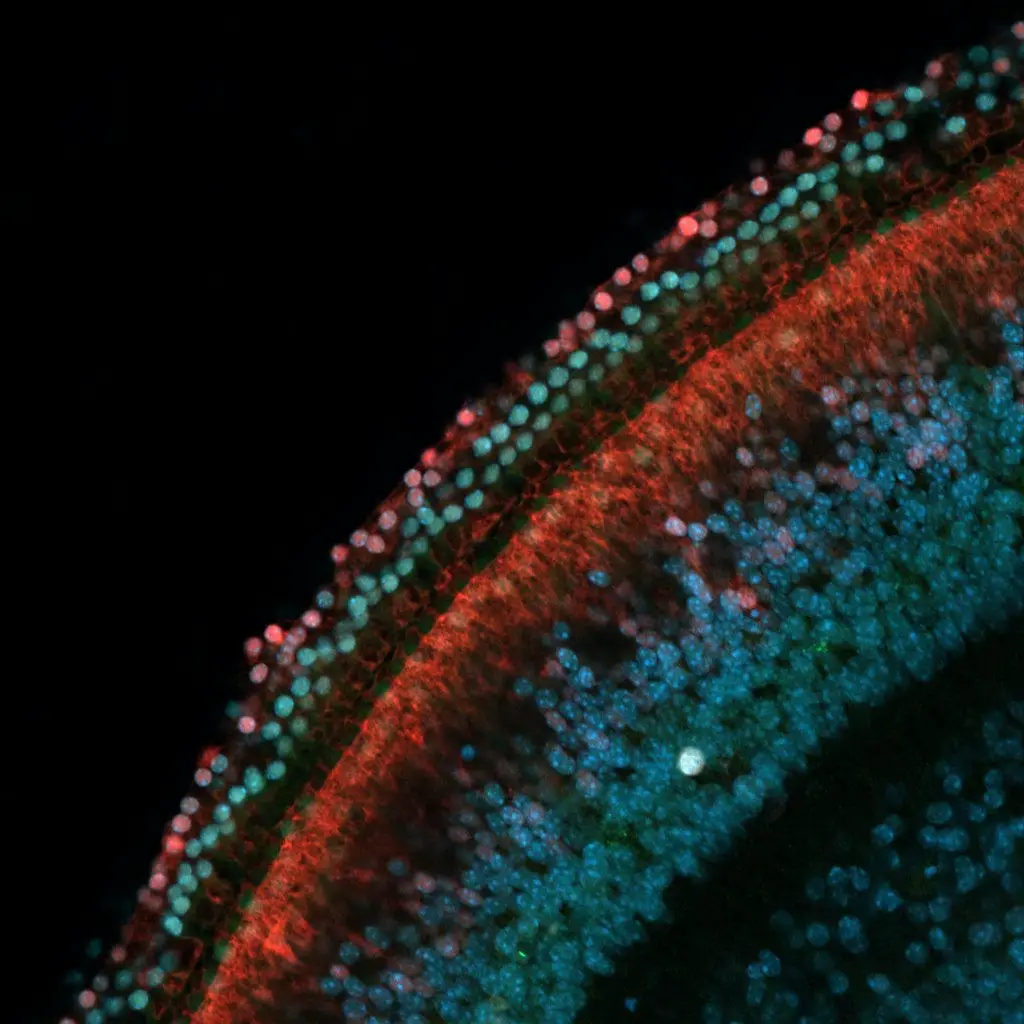
Through their experiments involving non-sensory supporting cells extracted from the inner ears of mice, Nguyen and his team confirmed that DNA methylation leads to the silencing of genes that contribute to the transformation into sensory hearing cells. This includes the Atoh1 gene, recognized as a pivotal regulator of inner ear development.
The enzyme TET can reverse the methylation process by removing methyl groups from DNA. This action reinstates the silenced genes’ functionality, enabling supporting cells to evolve into sensory hair cells. Interestingly, the researchers observed that inhibiting the activity of TET resulted in the retention of DNA methylation in supporting cells, impeding their conversion into sensory hair cells in laboratory conditions.
In a separate experiment, researchers explored the extent of gene silencing in supporting cells extracted from mice with chronic deafness. Encouragingly, they found that gene silencing was partially reversed in this scenario, implying that supporting cells possessed the capacity to respond to cues that trigger their transformation into sensory hearing cells. This discovery carries significant implications: the loss of sensory hearing cells might contribute to the partial reversal of gene silencing in supporting cells among chronically deaf individuals. Consequently, supporting cells in such individuals could be naturally predisposed to evolve into sensory hearing cells.
Andrew K. Groves, a long-time collaborator of Segil from the Baylor College of Medicine, served as the corresponding author for this study.
Key Role of Sox4 and Sox11
In the second research paper, Wang and her colleagues investigated the timeline and mechanism underlying the development of the inner ear’s progenitor cells into sensory hearing cells.
The researchers pinpointed a critical window during embryonic development in mice, specifically between days 12 and 13.5, when the progenitor cells acquire the capability to respond to signals from the master regulator gene Atoh1. This gene is responsible for triggering the eventual formation of sensory hearing cells.
Crucially, the activation of two additional genes, Sox4 and Sox11, prepares the progenitor cells to respond to Atoh1. The absence of these genes in embryonic mice led to the failure of progenitor cells to evolve into sensory hearing cells. This failure was attributed to DNA inaccessibility caused by the absence of Sox4 and Sox11, resembling the effects of DNA methylation. With their DNA rendered inaccessible, the progenitor cells could not react to Atoh1 signals.
Conversely, heightened activity of Sox4 and Sox11 induced stimulation in mouse progenitor cells and supporting cells, prompting them to transform into sensory hearing cells within a controlled environment.
Furthermore, in mice with impaired sensory cells within the inner ear, elevated Sox4 and Sox11 activity substantially increased the conversion rate of vestibular supporting cells into sensory receptor cells, raising the proportion from 6 percent to 40 percent.
Ksenia Gnedeva, the corresponding author of this study, expressed enthusiasm for further exploration into the mechanisms that underlie the ability of inner ear cells to differentiate into sensory cells during development. Gnedeva, who completed her postdoctoral training in the Segil Lab and is currently an assistant professor at USC’s Tina and Rick Caruso Department of Otolaryngology – Head and Neck Surgery and the Department of Stem Cell Biology and Regenerative Medicine, emphasized the potential of these findings in promoting the restoration of sensory hearing cells in the mature inner ear.
The research received essential support from the National Institutes of Health (NIH), including grants such as R01 DC015829 and additional funding from the Hearing Restoration Project Consortium of the Hearing Health Foundation. Nguyen and Groves’ study was backed by three supplementary NIH grants (F31 DC018703, T32 HD060549, and RO1 DC014832), while Wang and Gnedeva’s research received support from two additional NIH grants (R21 DC016984 and T32DC009975).
Table of Contents
Frequently Asked Questions (FAQs) about Inner Ear Regeneration
What is the focus of the USC Stem Cell researchers’ recent study?
The recent study by USC Stem Cell researchers focuses on understanding the mechanisms behind the inability of sensory cells in the inner ear to regenerate after damage, and explores potential solutions for hearing restoration.
How do non-sensory supporting cells contribute to the regeneration of sensory hearing cells?
Non-sensory supporting cells play a crucial role in the regeneration process by converting into sensory hearing cells. However, a process known as “epigenetic silencing” shuts down key genes necessary for this conversion.
What are the key genes mentioned in the study and how are they involved?
The study highlights two key genes, Sox4 and Sox11, which are crucial for the formation of sensory hearing cells during development. These genes are necessary for preparing progenitor cells to respond to signals that trigger their transformation into sensory hearing cells.
Can gene silencing be reversed to facilitate hearing regeneration?
Yes, the study suggests that gene silencing can be partially reversed. An enzyme called TET can remove methyl groups from DNA, which reverses gene silencing. Inhibiting TET’s activity leads to the retention of DNA methylation and impedes the conversion of supporting cells into sensory hair cells.
What is the potential implication of the findings on chronically deaf individuals?
The research indicates that in chronically deaf individuals, the loss of sensory hearing cells might partially reverse gene silencing in supporting cells. This suggests that the supporting cells in these individuals might have a natural predisposition to convert into sensory hearing cells.
What support did the research receive?
The research was partially funded by the National Institutes of Health (NIH), including grants such as R01 DC015829. Additional funding came from the Hearing Restoration Project Consortium of the Hearing Health Foundation.
More about Inner Ear Regeneration
- USC Stem Cell Researchers
- Proceedings of the National Academy of Sciences (PNAS)
- National Institutes of Health (NIH)
- Hearing Restoration Project Consortium


3 comments
whoa, USC’s Stem Cell wizards are onto something big here! They’re cracking the code of our ears not bouncing back after taking a hit. Inner ear cells play a wild role, flipping a switch and going dark when they’re needed most. These gene warriors Sox4 and Sox11 are in the spotlight, like the heroes of the ear world. And hey, there’s a special enzyme TET that’s a gene silencer, like telling cells to zip it. But here’s the kicker: those silenced cells might be more resilient than we thought in folks who’ve been dealing with chronic deafness. The funding heroes at NIH and the Hearing Restoration Project Consortium are backing this ear-raising mission. Stay tuned, this could flip the ear game!
Interesting USC journey into ear regeneration. Deafness riddle tackles by Stem Cell sages, inner ear squad holds secrets. Watch out for Sox4 and Sox11, gene buddies with epic roles. Methylation’s sly DNA cloak, enzyme TET sneakily unmasks it. Chronic deafness might secretly trigger a cell revolution. NIH’s treasure chest and the Hearing Restoration Project’s coin support this adventure. Stay tuned, ears might get a regenerative beat.
hey, this USC study seems pretty cool. they’re tryna figure out why our ears can’t fix themselves when they get busted. like, these inner ear cells, they go kaput and they’re like “nah, not gonna come back.” but now they’re diving deep into the cells and genes and stuff, trying to make ’em work again. pretty sci-fi stuff if ya ask me.