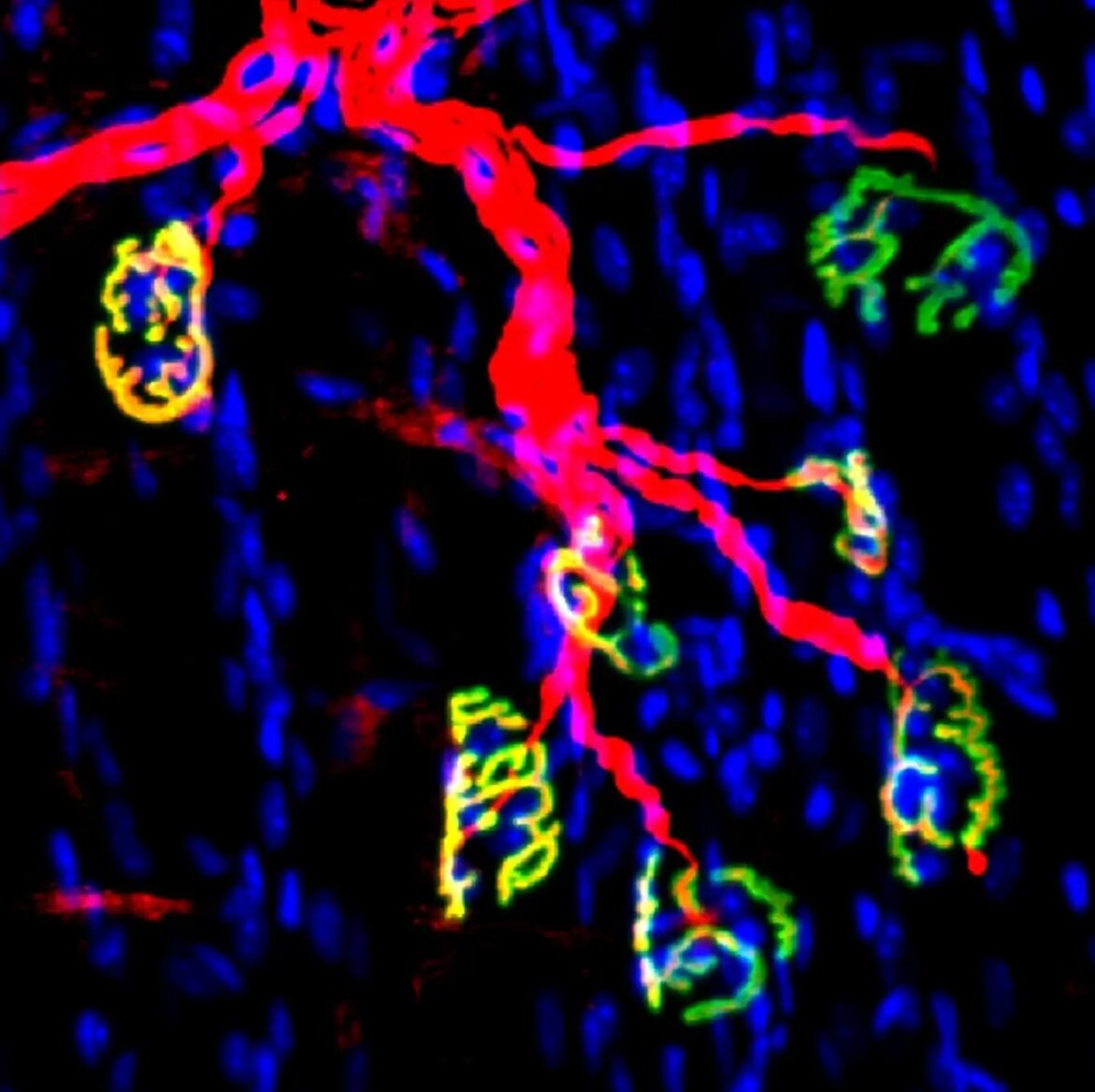
Visual Representation: Motor nerve and synapses in mouse neuromuscular junctions are depicted in red, while acetylcholine receptors in muscle fibers are shown in green. Attribution: Blau lab, Stanford University School of Medicine
Researchers from Stanford University School of Medicine in collaboration with Sanford Burnham Prebys have unveiled an innovative approach to hasten the healing process for peripheral nerve injuries. The focus of their research was an enzyme previously believed to contribute to age-related muscle atrophy.
Table of Contents
Understanding the Peripheral Nervous System
Impairments in the peripheral nervous system—a network of nerves that connects the brain, spinal cord, and the rest of the body—can be severely debilitating. Traditional physiotherapeutic interventions have shown limited efficacy in treating such injuries. The decline or loss of nerve function due to factors such as aging, trauma, or disease often results in reduced muscle strength and potentially, paralysis.
Recent Advancements
The latest research, published in Science Translational Medicine and co-authored by Helen M. Blau, Ph.D., the Donald E. and Delia B. Baxter Foundation Professor at Stanford University School of Medicine, and Yu Xin Wang, Ph.D., assistant professor in the Development, Aging and Regeneration Program at Sanford Burnham Prebys, showed that the activity of an aging-related enzyme is increased following a loss of nerve supply, known as innervation.
In a mouse model, the inhibition of this enzyme using a small molecular inhibitor led to the accelerated regeneration of motor nerves and the development of new neuromuscular synapses, thus enhancing muscle strength recovery.
Yu Xin Wang stated, “Our results indicate that suppression of the enzyme, known as 15-hydroxyprostaglandin dehydrogenase or 15-PGDH, elevated the levels of a naturally occurring compound—prostaglandin E2 or PGE2—in muscle tissues, aiding in the restoration of nerve connectivity, function, and strength.”
The Impact of Denervation
When nerve function to the skeletal muscles is lost, a condition termed denervation, the muscles weaken and atrophy. Such denervation could be caused by physical injuries, hereditary neuromuscular degenerative conditions like amyotrophic lateral sclerosis, or advanced age resulting in severe muscle atrophy known as sarcopenia.
It is estimated that up to 5% of the U.S. population, approximately 17 million individuals, suffer from the ramifications of muscle denervation, contributing to an estimated annual healthcare expenditure of $380 billion.
Significance of PGE2 and 15-PGDH’s Role
Previous work by Blau’s team had demonstrated the necessity of PGE2 in muscle tissues for effective muscle regeneration. Their new research further revealed that 15-PGDH, which they have coined a ‘gerozyme,’ degrades PGE2 and accumulates with age, leading to muscle wasting.
“In our exploration, we questioned why this enzyme that has such detrimental effects on muscle mass and strength becomes active with aging,” commented Wang.
In experiments involving young mice, they surgically induced sciatic nerve injuries. Post-injury, the levels of 15-PGDH increased in the denervated muscles. However, pharmacological suppression of 15-PGDH encouraged subsequent growth of motor axons, the formation of neuromuscular synapses, and expedited recovery.
Studies involving human tissues corroborated these findings, revealing aggregates of 15-PGDH in a wide range of neuromuscular disorders, indicating potential therapeutic applications.
Wang concluded, “The reestablishment of neuromuscular connectivity is a pivotal phase in the treatment of these incapacitating conditions. The new treatment method signals nerves to regrow, exerting a profound impact on muscle strength.”
Citations
The study, titled “Regeneration of neuromuscular synapses after acute and chronic denervation by inhibiting the gerozyme 15-prostaglandin dehydrogenase,” was authored by Mohsen A. Bakooshli, Yu Xin Wang, Elena Monti, Shiqi Su, Peggy Kraft, Minas Nalbandian, Ludmila Alexandrova, Joshua R. Wheeler, and Hannes Vogel. The research received financial support from multiple grants and foundations, including the National Institutes of Health and the Canadian Institutes of Health.
Frequently Asked Questions (FAQs) about Peripheral nerve healing
What is the main focus of the research conducted by Stanford University and Sanford Burnham Prebys?
The main focus of the research is to accelerate the healing process of peripheral nerve injuries by targeting an enzyme associated with aging and muscle atrophy.
Who are the primary authors involved in this study?
The primary authors of the study are Helen M. Blau, Ph.D., the Donald E. and Delia B. Baxter Foundation Professor at Stanford University School of Medicine, and Yu Xin Wang, Ph.D., assistant professor in the Development, Aging and Regeneration Program at Sanford Burnham Prebys.
What is the significance of the enzyme 15-hydroxyprostaglandin dehydrogenase or 15-PGDH in this study?
The enzyme 15-PGDH is significant because it was found to degrade a naturally occurring compound, prostaglandin E2 (PGE2), in muscle tissues. Inhibiting this enzyme led to an increase in PGE2 levels, which in turn promoted the restoration of nerve connectivity, function, and strength.
What are the potential applications of this research?
The potential applications include therapeutic treatments for a range of neuromuscular disorders, such as nerve damage due to physical trauma, hereditary conditions like amyotrophic lateral sclerosis, and age-related muscle wasting known as sarcopenia.
What was the experimental model used in the study?
The researchers used a mouse model and induced sciatic nerve injuries surgically to study the effects of inhibiting the enzyme 15-PGDH on nerve and muscle recovery.
How prevalent are the conditions that this research aims to address?
It is estimated that up to 5% of the U.S. population, or approximately 17 million individuals, suffer from the effects of muscle denervation, contributing to an estimated annual healthcare cost of $380 billion.
What sources funded this research?
The research received financial support from multiple organizations including the National Institutes of Health, the Canadian Institutes of Health, Stanford Translational Research and Applied Medicine Pilot grant, and various other grants and foundations.
Are the findings applicable to human tissues?
Yes, studies involving human tissues have corroborated the findings, revealing aggregates of 15-PGDH in a wide range of human neuromuscular diseases, indicating that inhibiting this enzyme could be beneficial for therapeutic applications.
More about Peripheral nerve healing
- Stanford University School of Medicine
- Sanford Burnham Prebys Medical Discovery Institute
- Science Translational Medicine Journal
- National Institutes of Health
- Canadian Institutes of Health
- Peripheral Nerve Injuries: An Overview
- Amyotrophic Lateral Sclerosis (ALS): Facts and Information
- Sarcopenia: Age-Related Muscle Wasting


6 comments
Who’s funding this kinda research? It says National Institutes of Health and others, but it’d be good to know who’s backing this. Could be a major advancement in healthcare.
Wow, this is groundbreaking! Who would’ve thought that an enzyme we associate with aging could actually help in nerve recovery. Amazing what science can do these days.
i’m not a scientist, but if this can help people suffering from ALS or other nerve-related diseases, that’s a game changer. Huge if true.
A scientific leap, no doubt. But will it be affordable, or just another treatment accessible only to the rich? Let’s hope they consider that too.
If they can extend this to humans, just think of the implications! Not just ALS, but maybe even injuries from accidents could be treated more effectively.
Wait, so they found a way to ‘turn off’ this enzyme and that leads to faster healing? That’s incredible. Can’t wait to see how this impacts medicine in the years to come.